
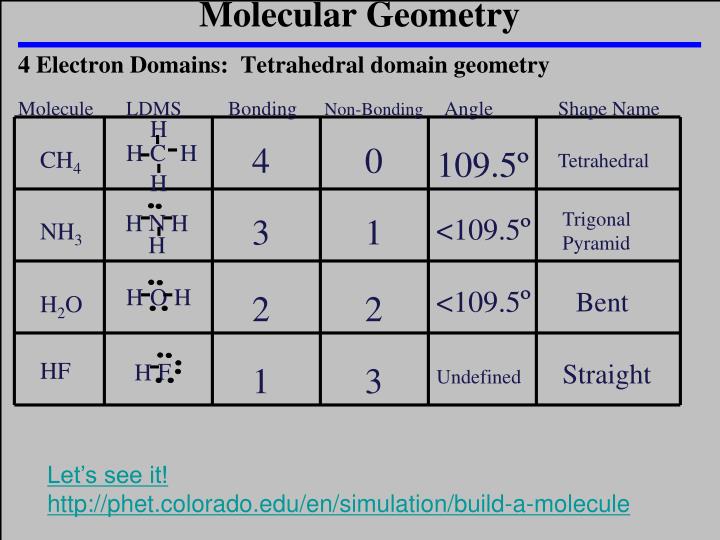
The formula to find out hybridization is:- H = N.A. To determine the C2H4 molecular geometry, it is required to find out the hybridization of C2H4. So we got to know that the molecule has no lone pair. The atoms that are attached to the carbon atoms = 4 L.P stands for the lone pair on the central atom, V.E stands for the Valence electron of that particular central atom, and N.A stands for the number of atoms attached to the central atom. We can also use the formula for the confirmation of lone pairs i.e. In accordance with the Lewis structure of C2H4, it is found that there is no lone pair present on the central atom. Firstly, discover the number of lone pairs that the central atom of C2H4 has.Īnother term for a lone pair is unshared pair. The three steps to find out the C2H4 molecular geometry and bond angles are:ġ.
#C2h4 electron domain geometry how to
How to find out the C2H4 molecular geometry and bond angles? AX3 defines the shape of C2H4 as a Trigonal Planar with 120 degree bond angles. As the molecule does not have any lone pairs, we may ignore N. So, we got AX3 for the C2H4 molecule by the A-X-N method. ‘N’ denotes the number of lone pairs attached to the central atom, and C2H4 has no lone pair, meaning N=0. Here, the two carbon atoms are bonded, and both atoms have two hydrogen atoms attached to them. The number of atoms that have bonded with the central atom is denoted by ‘X’. Here, the central atom is represented by ‘A’. The A-X-N method can find out the C2H4 molecular geometry and bond angles. Lewis structure helps to find out C2H4 molecular geometry as Lewis diagram determines the number of lone pairs and bond pairs a molecule comprises. The bonded pair of hydrogen attached to carbon repels each other, and as a result, the figure thus formed is a trigonal planar. In the case of C2H4, each carbon in its molecule undergoes sp² hybridization, and the two hydrogens make the structure look like a two-dimensional triangular planar. Molecular structure defines the arrangement of atoms in a molecule or ion. As per VSEPR theory, C2H4 molecular geometry and electron geometry are the same, i.e. Each of the sp² carbon atoms has a trigonal planar geometry and an angle of 120o between the bonds. Here, one 2p orbital does not change, and it will help form a pi bond. C2H4 is an example of sp² hybridization, meaning one s orbital, and two p orbitals are mixed to give three sp² orbitals. It is said to be a hydrocarbon that has two carbon atoms connected to it with a double bond. C2H4 is the chemical formula of a colourless and flammable gas known as Ethylene.


 0 kommentar(er)
0 kommentar(er)
